Introduction: There is limited data on SARS-CoV-2 vaccination responses in the rare hematological disorders paroxysmal nocturnal hemoglobinuria (PNH) and aplastic anemia (AA). These patients were expected to have more severe COVID-19 infection and reduced vaccination responses due to their underlying disease and immunosuppressive treatment. We previously reported limited anti-spike IgA/G/M responses after first COVID-19 vaccination which improved following second vaccination (Pike et al, Lancet Haematology 2022). Here we report spike-specific IgG, in vitro viral neutralization and T-cell responses in 244 patients with PNH and/or AA and in 49 healthy volunteers, following the first four vaccinations.
Methods: In 2021, the United Kingdom National PNH service centre based in Leeds initiated a prospective observational non-interventional study evaluating immune responses to COVID-19 vaccinations in patients with PNH and/or AA. All patients were consented to the Leeds PNH Research Tissue Bank. At baseline, the patient cohort comprised 94 patients with classic PNH, 75 with AA-PNH overlap and 75 with AA with asymptomatic PNH clones <50%.
Serological humoral responses were evaluated in blood samples using a quantitative spike-specific IgG SARS-CoV-2 ELISA (EuroImmun). Samples were further analyzed using a high-throughput live-virus neutralization assay performed at the Francis Crick Institute, London, against wild-type SARS-CoV-2 and against omicron B.1.1.529 and delta B.1.617.2 variants. Adaptive T-cell immune responses were assessed by incubating cryopreserved peripheral blood mononuclear cells with SARS-CoV-2 spike and nucleocapsid peptides in an ELISpot interferon-gamma release assay (Oxford Immunotec).
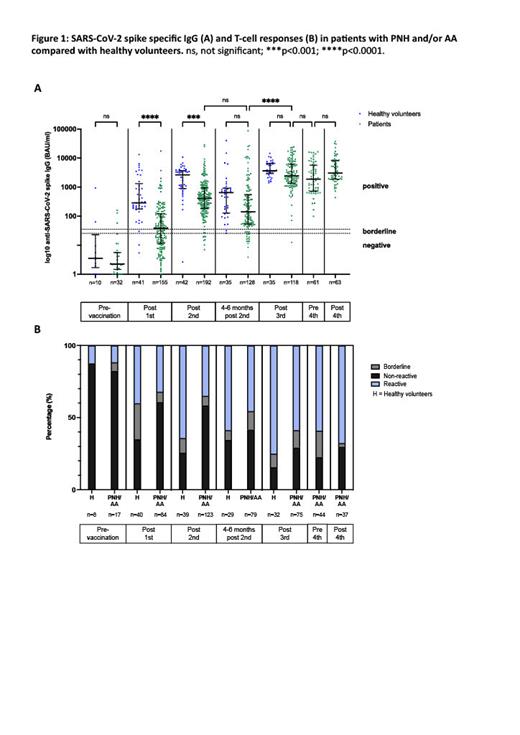
Results: Prior to vaccination (baseline) in-vitro anti-spike IgG response was not significantly different between patients and healthy volunteers (p>0.99). After first vaccination, 2/40 (5%) healthy volunteers and 66/155 (42.6%) PNH and AA patients failed to mount a detectible spike-specific IgG. The antibody titre was also significantly reduced (median spike-specific IgG titre in patients 37.9 BAU/ml [IQR 11.56-120.7] versus 289.4 BAU/ml in healthy volunteers [IQR 177.7-1326], p<0.0001). The magnitude of IgG response improved after second vaccination but remained lower than healthy volunteers (median IgG titre in patients 408.6 BAU/ml [IQR 184.1-942.6] versus healthy volunteers 2639 BAU/ml [IQR 894.0-3706], p<0.001). However, responses improved sequentially following repeated vaccinations and after the third dose were equivalent to healthy volunteers (p>0.99).
Viral neutralizing antibody activity elicited by vaccination showed similar results, with significantly reduced titres post first vaccination against wild-type SARS-CoV-2 in patients compared with healthy volunteers (p=0.0001). After second vaccination, neutralizing antibody titres in patients versus healthy volunteers remained significantly reduced against wild-type (p=0.0088) and the delta variant (p=0.0001) but not the omicron variant (p=0.078). Reassuringly, responses improved after 3 rd dose of vaccine following which there was no significant difference detected for all three strains (p>0.99).
Of the evaluable samples, anti-spike T-cell responses were reduced at all timepoints in patients compared with healthy volunteers but improved with each vaccination. After first vaccination, a reactive result was seen in 32.1% (n=27/84) versus 40% in healthy volunteers (n=16/40) and remained low after second vaccination (35.0% (n=43/123) in patients versus 64.1% (n=24/39) in healthy volunteers). However, after third vaccination, responses had improved to 58.7% (n=44/75) in patients versus 75% (n=24/32) in healthy volunteers. After 4 th vaccination, spike-specific responses were detectable in 67.6% (n=25/37) of patients. There was limited reactivity to nucleocapsid antigens in both groups at all timepoints highlighting that the observed responses were due to vaccination rather than COVID-19 infection. To date there have been no deaths due to COVID-19 infection in patients in this study or in our wider service population since the introduction of the vaccination programme.
Conclusion: This study demonstrates the importance of repeated SARS-Cov-2 vaccinations in patients with PNH and/or AA in order to improve humoral and cellular immune responses.
Disclosures
Pike:Sobi: Honoraria. Arnold:Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Florio: Honoraria. Munir:Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Muus:Novartis: Other: Advisory board member; Sobi: Other: Travel support and lecture fees. Griffin:Regeneron Pharmaceuticals: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees; Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees; Biocryst: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees; Apellis: Other: educational grant support . Hillmen:Apellis: Current Employment, Current equity holder in publicly-traded company. Kelly:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Biologix: Honoraria, Speakers Bureau; Astellas: Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal